So, as we all know Kilogram or kg is the SI unit for measuring mass. Kg is a more or less perfect standard to measure things that we need in our day-to-day lives. For things with a little less mass, like a fancy object or a little amount of food, we use grams. Moreover, for costly metals like gold, we mostly use grams or even smaller units like milligrams. 1 gram is one-thousandth of 1 kg. However, gram is a very big unit when we are talking about particles at the molecular or atomic level. We need to realize they are invisible to the bare eye. So, they can never have a mass that is at least 1 gm or 1 milligram. Therefore, in such cases converting grams to atoms becomes very important.
An atom consists of electrons, protons, neutrons chiefly. So, it is the most basic unit of an element. However, for a compound, the basic unit is a molecule. So, what happens is the same or different atoms combine with each other to form a molecule. Now, one has to measure molecular mass in daltons. However, finding the molecular mass on its own is nearly impossible. Think about it, your entire body weighs 60 kgs, out of that can you measure a single cell on your skin easily? No. Despite the fact that rigorous scientific calculations can appropriate a value, one can represent such masses best in relation to something bigger. This is therefore another important reason why conversion of grams to atoms and vice-versa is important.
We generally measure atomic and molecular mass with reference to the mass of Carbon-12 isotopes. However, before that, we need to know what exactly these terms mean. In the following sections, we will both define them and trace their relations.
Grams to atoms conversion
So, we have already seen why converting grams to atoms is extremely important. For carrying out every kind of scientific calculation in Chemistry, you need to convert grams to atoms. However, before all of that one must know what atomic mass is.
What is atomic mass?
So, atomic mass is the mass of an atom- as simple as that. However, it is also important to note that the nuclides that are protons and neutrons contribute mostly to this mass. Electrons, with negligible mass, occupying the shells hardly contribute any. Moreover, the numeric value of the atomic mass that you will get in daltons will be the same as the mass number of the atom. Now, the mass number of an atom is the total number of neutrons and protons present in its nucleus.
However, there is something called the relative isotopic mass too. You can get this by dividing the mass of the isotope by the atomic mass constant Mu. So, the atomic mass constant is the mass of a carbon C-12.

Now, what are isotopes?
Isotopes are atoms of the same element but they have different mass numbers. So, the relative isotopic mass of an atom has no dimension. For example, the atomic mass of Carbon-12 isotope is 12 Da but the relative isotopic mass is simply 12. Now, the atomic mass or the relative isotopic mass caters to a particular isotope of the element. However, in real life, elements are generally not isotopically pure. Therefore, we consider the elemental atomic mass. So, this is the average atomic mass of an element in regard to the many isotopes.
So, now that we know what atomic mass is, let us take a look at its units and the conversion formulas of grams to atoms calculator. Converting grams to atoms not only makes the calculation easier but also gives us an idea of the mass that is being considered. So, this is because we have an average idea of what a gram is. Therefore we can understand the mass of an atom with respect to it much better than we can via a completely alien unit like daltons.

Grams to atoms calculator
So, before we go into the details of the formula to convert grams to atoms, we must have a proper understanding of the units.
What is the atomic mass unit or amu?
So, the atomic mass unit (amu) or unified mass (u), or daltons (Da) is the unit scientists use most commonly for doing scientific calculations. So, 1 atomic mass unit or unified mass or dalton is defined as one-twelfth of the mass of a single carbon-12 isotope. However, you must remember that scientists do this measurement only when the C-12 atoms are at rest. So, the obvious question is what does at rest mean? Well at rest should ethically mean that mass of the atom is not getting converted into energy, via E=mc^2. Therefore, m is the nuclear mass, c is the speed of light in the vacuum that is 3 x 10^8 m/s, and E is the binding energy.
So, from here, you need to remember an important condition. Atomic mass is always a little less than the sum total of the masses of protons and neutrons inside the nucleus. So, this happens because of the binding energy mass loss.

How to calculate the number of atoms in a sample?
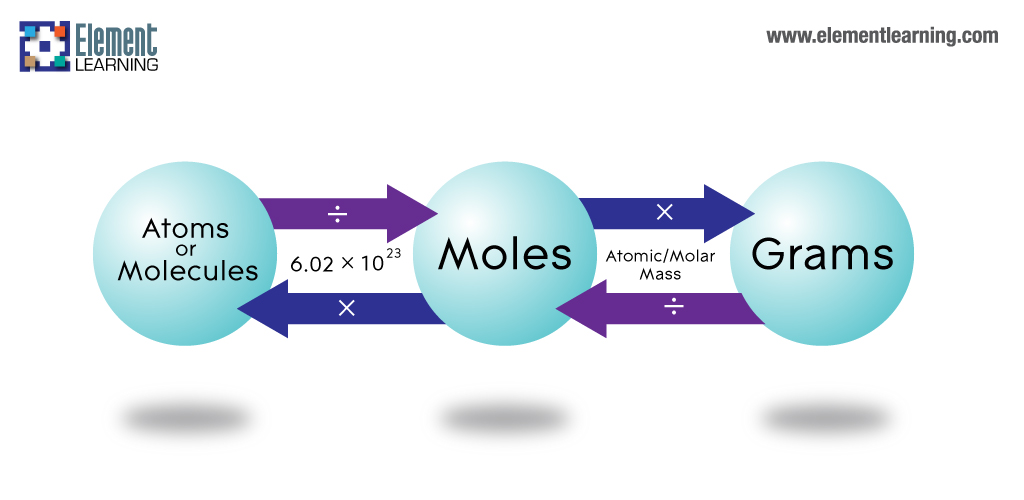
So, at first, you have to find out the atomic mass of an element. Therefore to do this, you have to sum up the total number of neutrons and protons present in the nucleus. Then you have to multiply the value of the mass number by 1 amu. We will see how to convert grams to atoms to get 1 amu in the next part. However, we also need to know the total number of atoms we have in a sample to calculate its mass. Therefore, to do this, you have to first weigh the total sample. So next you have to divide it with the atomic mass of that particular element and then multiply it with the Avogadro’s number. Now, Avogadro’s number is 6.02 x 10^23. Moreover, this is something you will need in all calculations of chemistry, including the conversion of grams to atoms.
Grams to atoms formula
So, we already know what an atomic mass unit is. However, we need to get into the most important part. This is how to convert grams to atoms or vice-versa via the atomic mass unit. So, the formula is-
1 amu = 1.661 x 10^-27 kg.
Therefore, 1 amu = 1.661 x 10^-27 x 10^3 gms
So, to convert grams to atoms-
1.661 x 10^-24 gms = 1 amu.
So, for any calculation involving amu where one needs to get the value in gms, one can simply multiply the value with 1.661 x 10^-24.
Now, looking back at the conversion of grams to atoms we need to remember-
1 Da or 1 amu is the atomic mass constant which we are basically multiplying to find the value in kgs or grams.
So, 1 amu = Molar mass constant/ Avogadro’s constant.
However, the molar mass constant that we use in converting grams to atoms is the molar mass of the C-12 isotope. So, this is the total mass of C-12 divided by the number of moles- 12. Moreover, Avogadro’s constant is 6.02214076 × 1023. So, it is the number of units that you can find in 1 mole of any substance.
Therefore, 1 amu = M (C-12)/ (12 x Avogadro’s number) = 1.661 x 10^-27 kg. So, in this process of converting grams to atoms, M is the molar mass of C-12. So, we must know what a mole or molar mass is.
Grams to atoms stoichiometry
So, stoichiometry is the relationship between the relative quantities of various substances that take part in a reaction or result from a reaction. So, converting grams to atoms or vice-versa is also a part of stoichiometric calculations.
What is a mole?
So, in chemistry, 1 mole is the Avogadro number of some chemical unit. However, this unit can be atoms, molecules, ions, or anything. Scientists use the mole because it makes calculations easier. Therefore, it acts as a common ground between atoms, molecules, or ions which are many in number. So, they are helpful in the conversion of grams to atoms too. Therefore, in simple words, it is exactly 6.02214076×10^23 of any elementary entities or particles.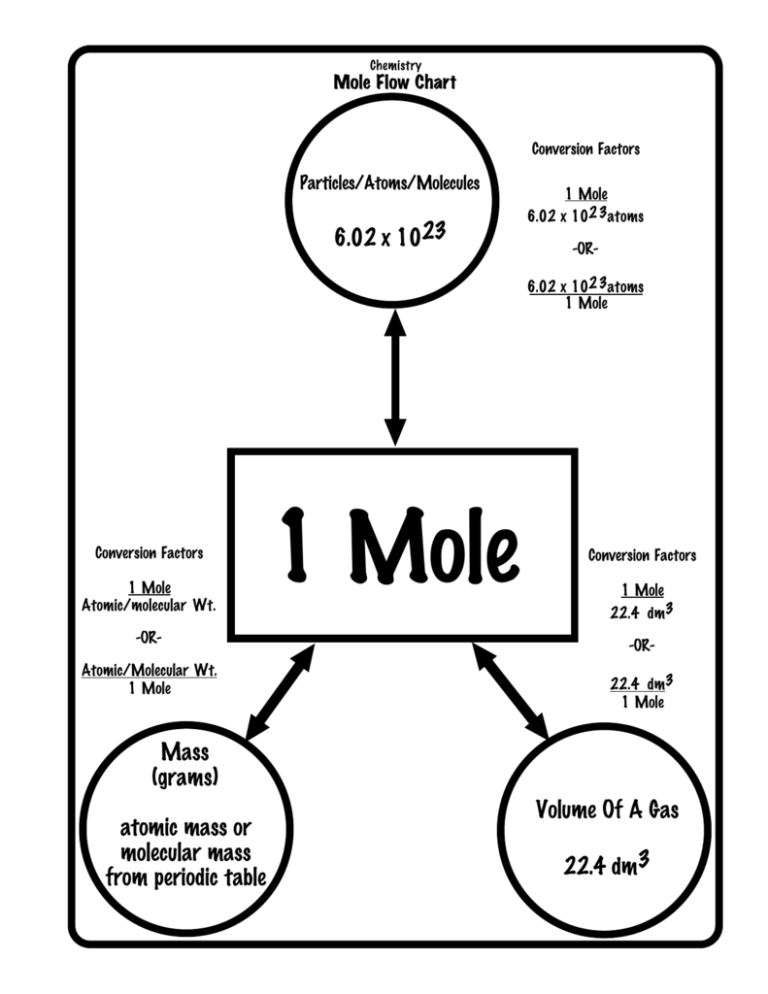 What is molar mass?
What is molar mass?
So, in chemistry scientists define molar mass as a ratio. This exists between the total mass of a substance and the number of moles that are present in the sample at hand. Moreover, the unit of molar mass is kg per mole. However, we know the value of 1 mole. Therefore, we can easily find the mass in kgs and hence in gms.
Therefore, all these calculations and definitions are mandatory for converting grams to atoms or atoms to grams. So, the grams to atoms calculator depends strongly on moles and molar mass which connect the two entities.
Grams to atoms to moles
Now, the amount of mass that you find in 1 mole is the same as the number of atoms in exactly 12 grams of pure carbon-12.
So, 1 mole by that calculation = number of atoms that are present in 12 g of C-12
- = 6.022 x 10^23 atoms. This, however, scientists have found experimentally. Moreover, this is also Avogadro’s number. It calculates the number of particles in 1 mole of the substance.
So, to convert grams to atoms to moles, you have to follow two steps. Therefore, if you see the derivation, you cannot directly go from grams to atoms. So, first, you convert the grams to moles. Therefore, to do this, you have to divide the total mass by the number of moles that are present. So, from here, to get the total number of atoms, one has to divide the molar mass by Avogadro’s number.
Therefore, you can well realize how you can absolutely do nothing without Avogadro’s constant. So, its value is 6.022 x 10^23.
Grams to atoms of a compound
So, when you are working with a compound, you need to remember that all the atoms are not the same. Therefore, you have to calculate at a molecular level. So now, you have to check the atoms that are present in the compound. Next to convert grams to atoms, or rather the other way around, you should calculate how many atoms of each element are there in 1 molecule of the compound. So, you multiply the atomic mass of each element with the number of atoms it has inside the molecule. Moreover, if you add all these values, you will get the molecular mass of the compound. Therefore, from here you can easily find the molar mass. So, you can go on with your conversion from grams to atoms.
Grams to atoms Calcium
So, the entire process of converting grams to atoms will be much easier if you follow a simple example. Therefore, here we are considering Calcium. Calcium is a metal. So, it is a monatomic element. Hence, as per the periodic table, it has an atomic mass of 40.1g. Moreover, it is monoatomic, meaning there is only 1 atom.
So, molar mass of Ca = 40.1 gm/mol
Therefore, moles in 1 gram of Ca = 1/ (40.1 gm/mol)
So, atoms in 1 gram of Ca = 1 gm/ (40.1 gm/mol) x 6.022 x 10^23 /mol
- = 0.1501 x 10^23.

Grams to atoms FAQs
Do more grams mean more atoms?
Well, it depends on the element that we are talking about. So, here the concept of moles comes in. Therefore, how many atoms there will depend on the atomic mass of the element. Hence, while calculating grams to atoms, the more the number of moles, the more are atoms.
How many atoms are there in a gram?
So, here again first of all you need to find out the number of moles present in the given amount of sample. From then, by multiplying Avogadro’s number, you can easily find the number of atoms present. Therefore it will differ with elements because each has a different atomic mass. This will give rise to a different number of moles in different elements for 1 gm of it. So, iron and calcium will have different numbers of atoms in 1 gm of iron or calcium respectively. You cannot convert grams to atoms in a generalized way.