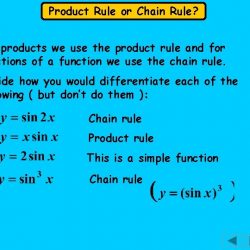
The Dimensional Analysis Calculator is a free online tool for determining the dimensions of two physical quantities. Dimensional analysis is the study of the relationships between different physical quantities by identifying their base quantities (such as length, mass, time, and electric current) and units of measure (such as miles vs. kilometres, or pounds too. kilogrammes) and tracking these dimensions as calculations or comparisons are performed in engineering and science. Because all units use the same 10-base, converting units from one dimension to another is typically easier in the metric or SI system than in others.
However, the factor-label approach, often known as the unit-factor method, is a widely used technique for such conversions utilizing algebraic rules. But our online dimensional analysis calculator tool speeds up the process by analyzing the two physical quantities in a matter of seconds.
Dimensional Analysis Calculator With Steps
The following is how to use the Dimensional Analysis calculator:
- Step 1:
However, firstly fill in the appropriate input fields with two physical quantities in the Dimensional analysis calculator.
- Step 2:
Then to get the analysis, click the “Submit” button.
- Step 3:
Finally, in the new window, our dimensional analysis calculator will show the dimensional analysis.
Dimensional Analysis Calculator Moles
Dimensional analysis calculator determines the number of moles in a substance of a given mass. However, this gram to mole calculator can quickly show you how to convert moles to grammes for any chemical. However, it can also be used to convert grams to moles in the opposite direction. Meanwhile, continue reading to discover the grams to moles formula, try solving a problem with how to convert grams to moles on your own, and never worry about converting g to mol again.
Dimensional Analysis Calculator Volume
Meanwhile, this Dimensional analysis calculator in the metric system may be used to convert: cubic meters to cubic feet gallons to litres cubic feet to gallons
Afterwards, convert cubic feet to litres, cubic feet to cubic meters, cubic feet to cubic inches, and litres to cubic feet.
Convert units between the American and British metric systems, as well as the standard metric system (SI).
However, various geometrical bodies can have their volumes determined. Meanwhile, these include a pyramid, a truncated pyramid, a cylinder, a hollow cylinder (pipe), a cone, a truncated cone, a sphere, a sphere segment, and a barrel. You’ll need to keep the units consistent. As a result, if you want the volume in cubic meters, you’ll need to enter all of the data in meters. The computations will be incorrect if you don’t.
Dimensional Analysis Calculator Medication
The dimensional analysis calculator approach employs a series of equivalency conversion factors from one measurement system to another, but it does not necessitate memorizing specific formulas. This method reduces errors and can be used to calculate all dosages.
- However, begin by determining the label or unit of measure that will be used in the answer.
- Then build the equation by putting information in the numerator that has the same label as the previous denominator. This will cancel out any unwanted labels. Continue until all units of measure that aren’t needed in the answer have been canceled out.
- Calculate to find the numeric answer that is accurately labeled. Wait until you obtain the final solution before rounding any numbers in the equation.
Let’s look at an example to show how this method works in Dimensional Analysis Calculator.
Example: IV Dose
Digoxin 0.5 mg IV should be given once a day. However, Digoxin 0.25 mg/mL is the medicine concentration accessible from the pharmacist. To give a 0.5 mg dosage, how much mL will you need?
- 1st step:
Firstly, what kind of unit of measurement (label) is required? On the left side of the equation, but this.
mL? =
- 2nd step:
Then place the information on the right side of the page with the same label as the numerator. We know that the medication concentration accessible in this case is 0.25 mg/mL. Meanwhile, in the numerator, write mL, and in the denominator, write 0.25 mg.
mL? = mL/0.25mg
- 3rd step:
However, the recommended dosage is 0.5 mg. Meanwhile, to cancel out the undesirable labels, enter information with the same label as the previous denominator into the numerator equation. Repeat this process until all undesirable labels have been removed.
mL? = (mL/0.25mg) × 0.5mg =
- 4th step:
Multiply the numerator by the denominator, then multiply the denominator by the numerator by the denominator. Meanwhile, to get the final solution with the correct label, divide the numerator by the denominator.
mL? = (mL/0.25mg) × 0.5mg = 0.5mL/0.25 = 2mL
Final answer: Suggest 2 mL of digoxin daily.
Dimensional Analysis Calculator Time
This formula is the quickest way to convert seconds to hours:
3,600 hours = seconds
The time in hours is calculated by multiplying the time in seconds by 3,600. The conversion ratio utilized in the formula is 3,600 seconds per hour since there are 3,600 seconds in one hour.
For example, using the technique above, you may convert 5,400 seconds to hours.
5,400 sec = (5,400 ÷ 3,600) = 1.5hour
The formula above can be used to find the hours in decimal form, but these procedures can be used to get hours, minutes, and seconds.
- To calculate the total hours, multiply the seconds by 3,600. Then, as the number of hours, use the number to the left of the decimal point.
- Then multiply the even hours above by 3,600 to get the remaining seconds. Meanwhile, subtract it from the total number of seconds.
- To get the total number of minutes left, divide the remaining seconds by 60. Then, as the number of minutes, use the number to the left of the decimal point.
- However, multiply the even minutes found above by 60 to get the remaining seconds. Subtract it from the total number of seconds. In short, this is how many seconds are left.
- Then fill in the form HH: MM: SS with the even number of hours, minutes, and remaining seconds.

Dimensional Analysis Definition
If a mass in grams was stated in milligram(10–3 g), it could be easier to work with. However, unit analysis, often known as dimensional analysis, is the process of converting between metric units.
Meanwhile, a method of proportional reasoning in which a given measurement is multiplied by a known percentage or ratio to produce a result with a different unit or dimension is known as unit analysis. However, we know from algebra that any integer multiplied by one remains the same. If the number has units and we multiply it by a ratio with units, the number’s units multiply and divide by the ratio’s units, yielding the original number (remember you’re multiplying by one) but with different units.
Read Also:Interval Notation Calculator – An Online Tool to Make Math Easy
However, this method can be generalized to find your answer by multiplying or dividing a given integer by a known ratio. The number given is a numerical value (with its units). The units in the denominator of the ratio match the numerator units of the given, and the units in the numerator of the ratio match either the next ratio or the final solution. When you multiply these together, you’ll get the correct units for your answer.
Dimensional Analysis Units
However, a unit is required to express the value of physical quantities. How else would we communicate to the merchant how much sugar, flour, oil, and other items we require? The number of physical quantities we measure, on the other hand, is enormous. However, consider how difficult it would be for us to recall all of them if each was given its unit.
Fortunately, however, most physical quantities may be expressed using only a minimal number of units. These are referred to as “basic units” or “essential units.” Meanwhile, the seven dimensions of the physical world are the physical quantities represented by these foundation units.
The following are the seven physical quantities, as well as their base units (in SI) and dimensions:
- Length: m (meter) [L]
- Then, Mass: kg (kilogram) [M]
- Time: s (second) [T]
- Then, Electric current: A (Ampere) [I]
- Temperature: K (Kelvin) [Θ]
- Then, the Amount of substance: mol (mole) [N]
- Then, Luminous intensity: cd (candela) [J].
We can also represent other quantities using these fundamental units. The area, for example, is determined as a product of the lengths of the sides, thus we can express it in m² units and dimensions of [L²]. Derived units are units that can be represented in terms of basic units.
Following units are some commonly derived units:
- Area: m²
- Then, Volume: m³
- Velocity: m/s
- Then, Acceleration: m/s²
- Density: g/mL or g/cm³
- Then, Force: kg× m/s², or the Newton (N)
- Then, Energy: N×m, or the Joule (J)
Dimensional Analysis Calculator unit Table
| Derived quantity | Symbol | SI derived unit | Dimension |
| Abasement | A | m⋅s | L T |
| Next, the Absorbed dose rate | – | Gy/s | L² T^−3 |
| Then, Acceleration | →
a |
m/s² | L T^−2 |
| Next, Angular acceleration | ωa | rad/s² | T^-2 |
| Then, Angular momentum | L | kg⋅m²/s | M L² T^-1 |
| Next, Angular velocity | ω | rad/s | T^-1 |
| Then, Area | A | m2 | L² |
| Next, Area density | ρA | kg⋅m^−2 | M L^-2 |
| Then, Capacitance | C | farad (F = C/V) | M^-1 L^-2 T⁴ I² |
| Next, Catalytic activity concentration | – | kat⋅m^−3 | L^-3 T^-1 N |
| Then, Centrifugal force | Fc | N⋅rad = kg⋅m⋅rad⋅s^−2 | M L T^−2 |
| Next, Chemical potential | μ | J/mol | L T^−5 |
| Then, Crackle | →
C |
m/s^5 | L^−2 I |
| Next, the Current density | →
J |
A/m² | L² T^−2 |
| Then, the Dose equivalent | H | sievert (Sv = m²/s²) | M L^−1 T^−1 |
| Next, Dynamic viscosity | v | Pa⋅s | T I |
| Then, Electric charge | Q | coulomb (C = A⋅s) | L^−3 T I |
| Next, Electric charge density | ρQ | C/m³ | L^−2 T I |
| Then, the electric displacement field | →
D |
V/m | M L T^−3 I^−1 |
Dimensional Analysis Calculator in Chemistry
Chemists and other professionals use dimensional analysis to transform units of measurement. Any unit can be converted to another unit of the same dimension. This means that we can convert a certain amount of seconds into another time unit, such as minutes because we know that one minute always contains 60 seconds. However, alternatively, we can convert a given mass in grams to kilograms, knowing that one kilogramme is always 1000 grams. Alternatively, we can convert lengths from kilometres to miles, although metric units are most commonly used in chemistry. However, it is sometimes possible to convert to a new dimension using a known conversion factor. One kilogramme of clean water, for example, is equal to one litre. However, you might convert a volume of water to a mass of water or vice versa using that knowledge.
Meanwhile, time, mass, length, and volume are some of the most commonly utilized dimensions in chemistry. The SI units, from the International System of Units, are the standard units of measurement (those used most commonly so that they may be easily communicated with other scientists). However, the SI unit of time is seconds, which is denoted by the letter s. Then kilogramme is the SI unit of mass. It’s written as kg, with the k standing for kilo and the g standing for gramme, the base unit of mass. The meter (symbol m) is the SI unit of length. Chemists also utilize moles, which is a SI unit for measuring the amount of a chemical. However, a mole is made up of approximately 6.022 x 1023 particles (atoms or molecules). The symbol for it is mol.
Dimensional Analysis in Physics
You’ll frequently be asked to determine the numerical value and units of a variable in an equation when working on physics problems. The numerical value is usually not difficult to obtain, however, the same cannot be said for the units for a beginner. However, dimensional analysis is a handy way for obtaining the units of a variable in an equation, and this self-instruction unit covers it.
Meanwhile, the majority of physical quantities may be stated in terms of five basic dimensions. However, the Greek letter theta () represents mass (M), length (L), time (T), electrical current (I), and temperature (T). These five dimensions were chosen as the foundation because they are simple to quantify in experiments. Units and dimensions are not the same things.
Dimensional Analysis to Remember Equation
The physical amount of speed, for example, might be measured in meters per second, miles per hour, or other units. However, because speed is always a length divided by time, regardless of the units employed, we refer to the dimensions of speed as length divided by time, or simply L/T. Similarly, because the area can always be calculated as a length, the dimensions of the area are L². Although the area of a circle is commonly represented as πr², we could also write it as
πr (which is a length) × r (another length).
Let’s imagine you’re not sure whether time equals speed/distance or time equals distance/speed.
However, we may double-check this by ensuring that the dimensions on both sides of the equations are the same.
On each side of the equation, by reducing both equations to their fundamental units, we get
[T] = [ LT^(-1) / L ] = [T^(-1)] (Wrong)
[T] = [ L / LT^(-1) ] = [T¹] (Right)
Dimensional Analysis Calculator Practice
Follow these steps while solving dimensional analysis problems:
- Recognize the given (see the previous concept for additional information).
- Then determine the conversion factors that will assist you in getting from your original units to the unit you desire.
- Set up your equation so that the unwanted units cancel out, leaving you with the desired units. When a unit appears in both the numerator and the denominator of an equation, it cancels out.
- Now, to get the final result, multiply all of the numbers together. Remember to include the units and sig figs.
Dimensional Analysis Examples
Example 1
You may do the following dimensional analysis if you had a sample of a substance with a mass of 0.0034 grams and wished to express that mass in mg. However, the supplied quantity is 0.0034 grams of mass. Meanwhile, the mass in mg is the quantity you’re looking for, and we know that 1 mg = 10-3 g. One milligram per a tenth of a gram, or 1000 mg/1 gram, is the proportion or ratio.
As a result, 0.0034g x (1 gram/1 gram) = 3.4 mg
Example 2
How many hours are in 3 days?
Ans.
- Recognize the following: three days
- Determine the following conversion factors to assist you in getting from your original units to your desired unit:
24 hours/ 1day
- Set up the equation so that the unwanted units cancel out, leaving you with the desired units:
- 3 days × (24 hours/ 1day)
- To reach your final answer, multiply the equation. The final answer is 72-hour.
Example 3
How many inches are in 5 feet?
Ans.
- The provided measure is 5 feet.
- The conversion factor is: 12 inches / 1 feet
- Now set up the equation:
5-foot × ( 12 inches / 1 feet )
- Multiply through: 60 inches
30 inches equals how many feet?
Ans.
30 inches × ( 1 feet / 12 inches ) = 2.5 feet
Some Frequently Asked Questions about Dimensional Analysis Calculator
What is dimensional analysis?
When there isn’t enough information to put up accurate equations, a method of analysis in which physical quantities are described in terms of their fundamental dimensions is frequently used.
What is the principle of Homogeneity of dimension?
The concept of homogeneity states that each of the terms in a dimensional equation has the same dimensions on both sides.
How many kilograms go into a gram?
0.001 kilos equals 1 gramme (g) (kg).
What is a dimensional analysis and why is it used?
Dimensional analysis is a technique used in the physical sciences and engineering to reduce physical quantities like acceleration, viscosity, and energy to their basic dimensions of length (L), mass (M), and time (T) (T).