Sulfur dioxide (SO2) is a sulfur and oxygen-based gaseous air pollutant. Moreover, SO2 is a poisonous, heavy, and colorless gas and emits the odor of burned matches. When sulfur-containing fuels like coal, oil, or diesel undergo combustion, SO2 is produced. Besides, one can also create it as a by-product of copper extraction. Additionally, volcanic activity can also result in the release of sulfur dioxide naturally.
The smell of sulfur dioxide is similar to that of nitric acid. It can interact with water vapor in the atmosphere to generate sulphuric acid. Sulfur dioxide can also be converted to sulfates in the atmosphere. Nonetheless, sulfate is a major source of fine particle pollution in the eastern United States.
Occurrence of SO2
Sulfur dioxide is present on the Earth in very small proportions in the atmosphere – at about 1 ppm.
Further, one can find SO2 in varying amounts on many planets. Moreover, the most significant among them is the Venus atmosphere, which has a 150 ppm concentration of SO2. Besides, it is also the third-most significant atmospheric gas in Venus. It condenses there, forming clouds. Thus, it is an important component of chemical reactions in the planet’s atmosphere, contributing to global warming.
Also, when it comes to the Galilean moons, SO2 is thought of existing as a block of ice. Likewise, it is assumed as subliming frost or ice on Io’s trailing hemisphere. Also, it is available in the crust and mantle of Europa, Callisto, and Ganymede. Perhaps, it might also be found in liquid form, therefore reacting quickly with water.
Preparation of SO2
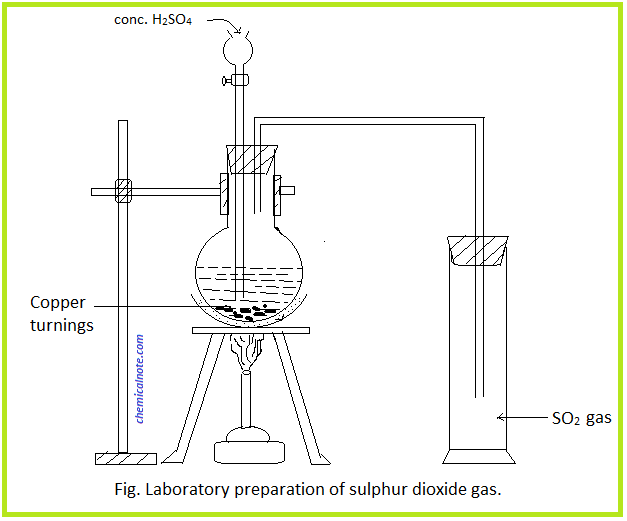
Now, we shall list a few methods of laboratory preparation of SO2.
- Sulfur dioxide can be prepared by the reaction of metallic sulfite or metallic bisulfite with any dilute acid. Take the example of a reaction between dilute sulphuric acid and sodium sulfite. Therefore, this will yield SO2.
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2 - We can also obtain sulfur dioxide commercially as a by-product of the roasting of sulfide ores. The resulting gas is then dried, liquefied, and then stored in steel cylinders.
4FeS2 (s) + 11O2 (g) → 2Fe2O3 (s) + 8SO2 (g) - Sulphur dioxide is formed as a byproduct during the manufacture of calcium silicate cement.
2CaSO4 + 2SiO2 + C → 2CaSiO3 + 2SO2 + CO2 - In the same vein, we can obtain sulfur dioxide when hot concentrated sulfuric acid reacts with copper turnings.
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
Properties of SO2
The characteristics of sulfur dioxide are as follows:
- It is a colorless gas with a pungent smell of rotten eggs.
- Besides, it has high water solubility.
- It gets easily liquefied.
- Since SO2 dissolves in water to generate sulfurous acid, it has an acidic nature.
H2O + SO2 → H2SO3 - It is neither combustible nor supports combustion.
- It is a powerful oxidizing agent.
2H2S + SO2 → 3S + 2H2O - Likewise, it also acts as a reducing effect.
SO2 + 2H2O → H2SO4 + 2H
Hybridization of SO2
In the case of SO2, the electronic configuration of the ground state is 1s2 2s2 2p6 3s2 3p4. 1 electron from the 3px orbital jumps to the 3d orbital when in an excited state. As a result, we have 3p3. Further, the 3s2 and 3p3 now unite to create sp2 hybridization. This, in turn, has 3 equivalent orbitals with 2 paired electrons and 2 unpaired electrons.
Sulfur requires 2 unpaired electrons from the sp2 hybridized orbitals to form 2 sigma bonds with oxygen atoms. The remaining 2 paired orbitals form the lone pair of sulfur. Likewise, there are 2 more electrons of 3p that do not take part in hybridization. While one of them is a 3p orbital electron, the other one is a 3d orbital electron. These two conversely form the pi bonds between sulfur and oxygen.
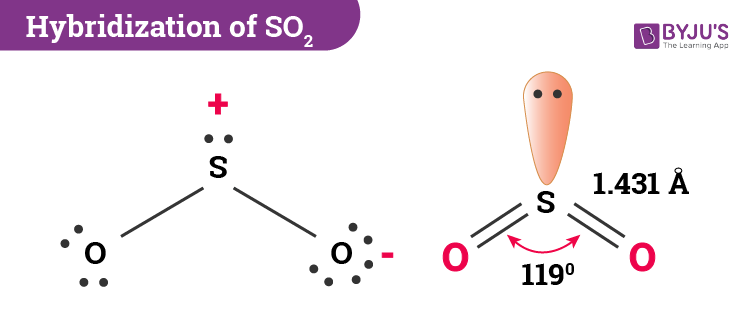
Therefore, SO2 has sp2 hybridization.
Molecular Geometry of SO2
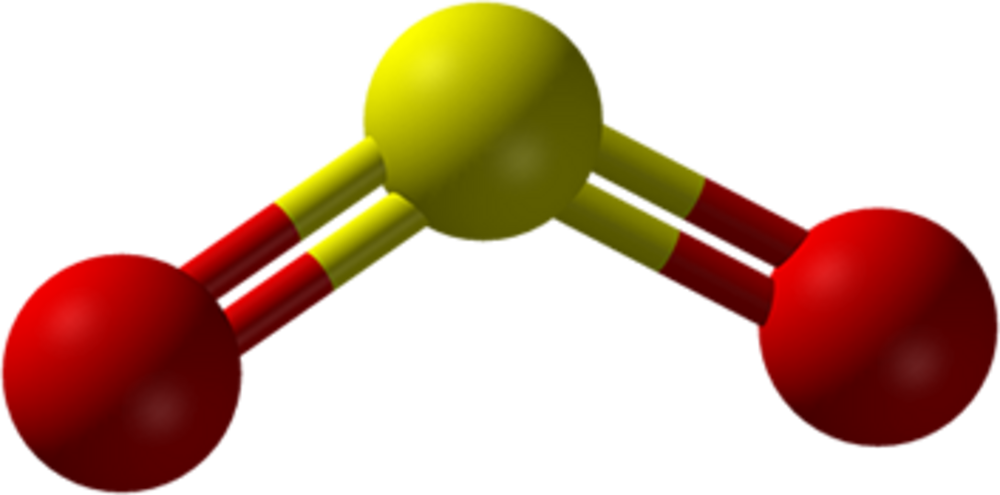
SO2 has a bent molecular geometry, with a bond angle of 120°.
Nonetheless, to determine the actual molecular geometry of SO2, we must follow a few steps at first. Primarily, comprehend the distribution of electrons between sulfur and oxygen. Sulfur has 6 electrons on the outer orbit, while oxygen has four, with one electron assigned to each bond. So there are 10 electrons in total in 5 pairs. Further, we require 4 pairs to form bonds, therefore 1 pair remains alone. The 2 double bonds use 2 pairs of electrons each and thus, form a single unit.
Henceforth, we can say that the molecular shape of SO2 is V-shaped or bent. Moreover, we don’t consider the single lone pair in the shape’s description. As a result, our first impression of the original structure differs from reality.
Electron Geometry of SO2
The electron geometry of SO2 is in the shape of a trigonal planar. There are 3 pairs of bonding electrons arranged in a plane at a 120° angle. When 2 double pairs bond, they produce a bent shape since one pair remains alone.
Bond Angle of SO2
The bond angle of SO2 is 120°. 1 atom of sulfur shares a covalent bond with 2 atoms of oxygen. As a result, it causes electron pairs to repel each other, thus resulting in a 120° angle.
Molecular Orbital Diagram of SO2
We can see that the AO of sulfur on the left associates with the AO of oxygen on the right.
Notably, all the orbitals are full of 18 electrons, following the proper rule. Certain non-bonding orbitals are also present there. Furthermore, in the case of SO2, the antibonding orbitals are unoccupied.

Therefore, this completes the explanation of SO2‘s molecular orbital diagram.
Lewis Structure of SO2 Molecule
Firstly, to create the Lewis structure for the SO2 molecule, we need to arrange the 8 valence electrons on sulfur. Further, we need to compute the formal charge of each atom to create the optimum Lewis structure. You must already be aware that sulfur and oxygen – both have 6 valence electrons. With 2 oxygen atoms, there will be a total of 18 valence electrons.
- Let’s place sulfur at the center and oxygen atoms outside.
- Now, place the pair of electrons between all the atoms to form bonds.
- Finally, calculate the formal charges (FC) for each.
For Oxygen,
No. of valence electrons = 6
No. of bonds = 2
Lone pairs = 2
So, FC = 6-2-(2×2) = 0
For Sulphur,
No. of valence electrons = 6
No. of bonds = 2
Lone pairs = 2
So, FC = 6-2-(2×2) = 0
- Now, let’s finish the octet with the most electronegative element, O, to complete the structure. Each atom of oxygen will have a double bond and a single lone pair.
- We’ll now place the remaining valence electrons on the central atom to complete the structure. Since there are 4 bond pairs and 4 lone pairs here, the total number of electrons used – (4+4) x 2 = 16. As a result, the number of valence electrons remaining is 18-16 = 2. These electrons will be attached to the sulfur atom.
Henceforth, the final Lewis structure of sulfur dioxide is as follows:

Polarity of SO2
Not only should we consider the types of atoms in a molecule, but also the structure of the molecule. And that’s how we can find whether a molecule is polar or not.
To begin with, it is necessary to understand that oxygen-sulfur bonds are slightly polar. It is due to oxygen having a higher electronegative potential than sulfur. Likewise, this indicates that oxygen exerts a stronger pull on sulfur dioxide’s covalent bonds. Sulfur is present in the center of the molecule, in connection with the oxygen via bent bonds. Subsequently, this indicates that one side (top or bottom) of the molecule has both oxygen atoms on it. And thus, this gives sulfur dioxide a slightly negative charge. However, the other half of the molecule with sulfur provides it a slightly positive charge. As a result, SO2 is a polar molecule.
Uses of SO2 in Daily Life
Henceforth, let us take a look at the multiple uses sulfur dioxide serves.
- It is widely used in the food industry as a preservative. Moreover, one can use it to preserve products such as dried apricots, dried figs, and dried grapes too.
- It acts as a bleaching agent as well as a disinfectant to remove excess chlorine.
- In cold storage plants, it serves as a refrigerant.
- Additionally, in the laboratory, we can use it as a solvent as well as a reagent.
Effects of SO2
On the Environment
When sulfur dioxide reacts with water and air, it leads to the formation of sulfuric acid. As many of us already know, it is a major component of acid rain. Acid rain can cause the following:
- Deforestation
- Acidification of streams, which harms aquatic life
- Corrosion of building materials and paints
There is a less heavy industry in Queensland than in Europe or North America. It is because sulfur dioxide emissions there have a higher potential for generating acid rain.
On Human Life
Sulfur dioxide causes severe irritation to the eyes. Eye exposure to liquid sulphur dioxide can result in serious burns and vision loss. It even leads to severe burns on the skin. Headache, general discomfort, and anxiety are some of the other side effects.
Read Also: Glycolysis – Where What And How Does It Occur?
In addition, it has adverse effects on the respiratory system, particularly lung functioning. Sulfur dioxide affects the respiratory tract, which increases the risk of infection. Coughing, mucus secretion, throat irritation, and asthma, and chronic bronchitis are all aggravations by it.
More Facts about SO2
Therefore, in conclusion, let us have a final look at some additional facts about sulfur dioxide.
- Molar mass = 64.066 g mol−1
- Density = 2.6288 kg m−3
- Melting Point = -72°C, -98°F, 201K
- Boiling Point = -10°C, 14°F, 263K
- Water Solubility = 94 g/L
- Vapour Pressure = 237.2 kPa
- Acidity = 1.81
- Basicity = 12.19
- Magnetic susceptibility = -18.2·10−6cm3/mol
- Viscosity = 12.82 μPas
- Dipole moment = 1.62D
- Standard Molar Entropy = 248.223 JK−1mol−1
- Standard Enthalpy of Formation = -296.81 kJmol−1
- Coordination geometry – Dihedral