The atom’s electron shape could be very essentials it tells us approximately an atom’s reactivity, and bodily houses as well. In this lesson, we can cowl the floor country electron configuration, which determines the electron’s shape.
Electron Configuration Defined
Just like we’ve got an area to live, within side the international of subatomic particles, electrons additionally have their very own place. Where they cross is dictated through the electron configuration, which describes the electron association inside an atom.
For electrons, there may be a floor country and an excited country. In this lesson, we’re going to be discussing the floor country electron configuration. When we speak approximately the floor country electron configuration, we are speak me approximately the electron configuration of an atom at the bottom electricity degree feasible.
In the universe, the herbal country of a gadget is to apply the bottom electricity feasible, so for an atom’s electrons on the floor country, the electron association might be at the bottom electricity country this is feasible for that atom.
SPDF Notation
The periodic desk is split into s, p, d and f blocks. The s, p, d and f subshells have a most quantity of electrons related to them, as visible on this desk.
The numbers in the front of the s, p, d, and f blocks at the periodic desk characterize the electricity tiers. Let’s study the factors sodium (Na), aluminum (Al) and potassium (K). Their positions are indicated at the periodic desk visible here.
If we rely all of the manner from left to right, and right all the way down to wherein the factors are positioned at the desk, we get the configurations .
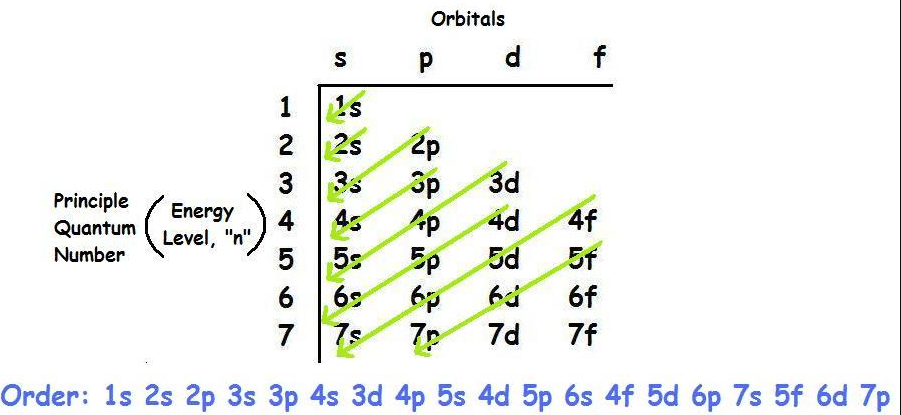
Orbitals
In addition to having specific electricity tiers, orbitals additionally have specific shapes and orientations, and every may be occupied through electrons. For every essential quantum quantity, n, there may be one s orbital, 3 p orbitals, 5 d orbitals and 7 f orbitals. Therefore, an s orbital can preserve electrons, a p orbital can preserve six electrons, a d orbital can preserve ten electrons, and an f orbital can preserve 14 electrons.
Ground State Electron Configuration
Quantum numbers
There are 4 quantum numbers n, l, ml, and ms. The essential quantum quantity n is a fantastic integer (1,2,3,4) and it represents the electricity of the orbital. The angular momentum quantum quantity l, is from zero to n – 1. The l values of zero, 1, 2, and three correspond to the s, p, d and f orbitals, respectively. The magnetic quantum quantity ml stages from –l to +l. This quantum quantity dictates the orbital orientation, along with px, py, or pz. The quantum spin quantity ms, is either +1/2 or -1/2 and it dictates the electron spin.
Hund’s Rule
Hund’s rule states that once stuffed sub-tiers apart from s orbital, electrons should now no longer be spin paired within side the orbitals till every orbital carries one electron, and no orbital may have electrons with the equal spin (ms).
Pauli Exclusion Principle
Pauli Exclusion Principle states that no electrons may have the equal quantum numbers. An orbital can most effective preserve zero, 1, or 2 electrons. They should have contrary spins if there are 2 electrons withinside the orbital.
Periodic Trend
Valence electron shells within side the periodic desk observe a trend. This may be known as the s block, the p block. The d block and the f block (lanthanides and actinides) which means that. In its floor country, an detail in a certain “block” can have its valence electrons. Within side the s, p, d, or f orbitals depending.