Carbohydrates are one of life’s four fundamental macromolecules. They contain monomers and polymers as building blocks. Monosaccharides are a type of polymer-forming monomers. Simple sugars, such as glucose and fructose, are the building components.
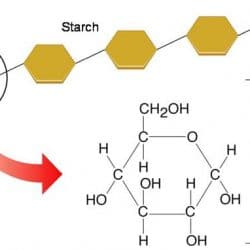
A disaccharide is made up of two monosaccharides joined together. Glucose and fructose bind together in sucrose (table sugar), for example. The term “Polysaccharide” also refers to large chains of monomers that are linked together. Starch is a polysaccharide loaded with hundreds of glucose molecules bound together. This can be also available in foods like pasta, bread, and potatoes.
Polymers Of Carbohydrates play a major account in the field of glycoscience. This covers the study and exploitation of polysaccharides. They have a current or potential function in bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, and wood, to name a few. We should provide essential structural information for all polysaccharides. This includes those obtained from a supplier that will disrupt their behavior in the ensuing study.

What are Monomers and Polymers
A monomer is a single molecule in organic chemistry. The term “monomer” refers to a single component. A glucose molecule, a nucleotide, or an amino acid are examples of organic monomers. Polymers are macromolecules made up of two or more monomers joined together. Polymer is a term that implies “many components.”
Polymers are usually the creation of lengthy chains of monomers. Starch, for example, is a polymer. It’s a combination of a lengthy string of glucose molecules. Proteins are polymers made up of amino acid chains. DNA is a nucleotide polymer that is extremely lengthy. Polymers include even small macromolecules like dipeptides (two amino acids linked together).
How do Monomers and Polymers relate to Carbohydrates?
Carbohydrates are the most generous biomolecules on the planet. Sugars and starches are examples of carbohydrates. Carbon, hydrogen, and oxygen must all be present in any carbohydrate. Furthermore, the proportions of C, H, and O are always 1:2:1. The symbol CH2O is commonly used to indicate this ratio. The name “carbohydrate” stems from the fact that the molecule is a creation of carbon and a water molecule (C – H2O), with “hydrate” referring to water. The suffix –ose (e.g., glucose, sucrose, cellulose, etc.) is typically used to identify carbohydrates.
Read Also: SO2 – A Detailed Discussion
Carbohydrates are called saccharides in biochemistry, from the Greek saccharin, which means sugar, but not all saccharides are sweet. Monosaccharides, or simple sugars, are the most basic carbohydrates. As detailed further in this essay, they are the building blocks (monomers) for synthesizing polymers or complex polysaccharides.
Carbohydrate Polymer Types
The amount of carbons in a monosaccharide determines its classification. Triose (three carbons), tetrose (four carbons), pentose (five carbons), and hexose (six carbons) are examples of general categories identified by a prefix. The prefix indicates the number of carbons and the suffix –ose, which indicates a saccharide; for example, triose (three carbons), tetrose (four carbons), pentose (five carbons), and hexose (six carbons). The most prevalent monosaccharide in nature is hexose D-glucose. Galactose is there to form the disaccharide milk sugar lactose. The fruit sugar fructose is two more prevalent and plentiful hexose monosaccharides.
Monosaccharides:
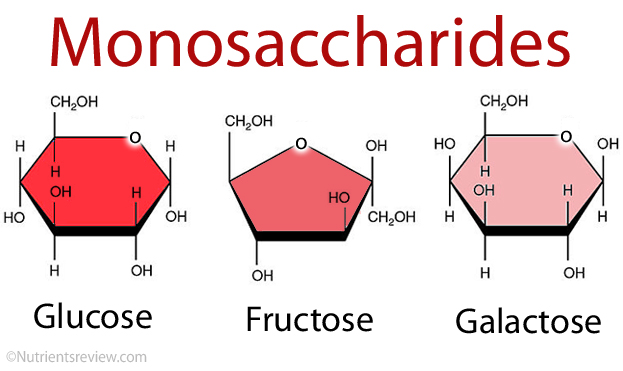
Monosaccharides are carbohydrate monomers and are typically referred to as “simple sugars.” There are dozens of them. However, they are divided into groups based on the number of carbon atoms they possess. Glucose, for example, also has six carbons and belongs to the hexose sugars group of monosaccharides: “hex” means “six.” Glyceraldehyde, for example, is a triose sugar with three carbons. Pentose sugars, such as ribose, have a molecular weight of 5. Monosaccharides have three to seven carbon atoms.
We’ll also concentrate on three hexose sugars that organisms use as fuel:
Glucose, fructose, and Galactose are the three sugars. Glucose, commonly known as dextrose, is also a simple sugar. C6H12O6 is the empirical formula. Glucose is the primary source of energy. For the rest of our life, It’s commonly referred to as blood sugar. Some of our body’s organs are fueled by Our brain. For example, it is made entirely of glucose. Fructose is the same as glucose. C6H12O6 is the same empirical formula as glucose. Fructose is also popular as fructose. Levulose is a carbohydrate that is similar to levulose. Fructose is sweeter than the other two hexose sugars. Fruits and honey contain a lot of it. Because fructose is sweeter, it is usually there in smaller amounts in food. As a result, it gets less expensive.
Disaccharides:
Disaccharides are two monosaccharides that are covalently there together. A glycosidic linkage is a name for this type of covalent bond. Disaccharides create themselves in a process called dehydration synthesis. Sucrose, maltose, and lactose are the three disaccharides we’ll look at.
Maltose is a polymer of two glucose molecules joined together. We can also create it by germinating grains as their starchy endosperm breaks down. When we manufacture beer and whiskey, we feed this disaccharide to the yeast.
Lactose is a sugar found in milk that is full of glucose and Galactose. Therefore, Lactose intolerance affects the majority of the world’s population (70 percent ).
It is There because of a shortage of lactase. Lactase breaks down sugar in the small intestine. Lactose functions as a sponge in the large intestine. This allows water to accumulate and cause diarrhea and abdominal cramps. Dairy-sensitive people can now drink milk by taking a lactase tablet, thanks to recent discoveries.
Oligosaccharides:
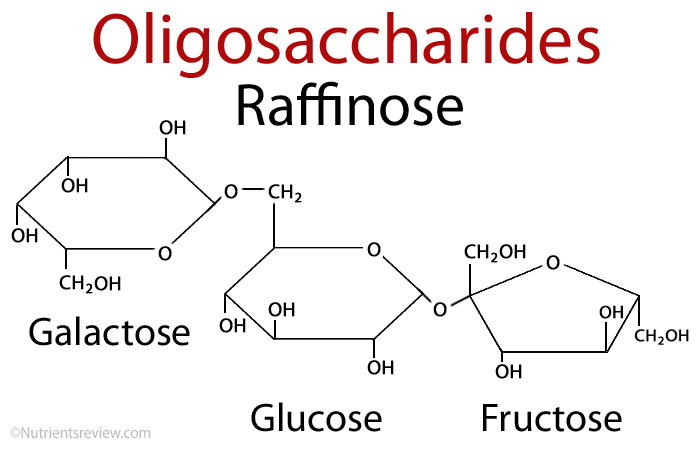
The word “oligo” signifies “few.” Raffinose and stachyose, for example, are oligosaccharides.
Monosaccharide chains are short chains of monosaccharides. Raffinose is there in the diagram below. Sucrose and Galactose are on the left. At the same time, stachyose (sucrose and glucose) is on the right.
On the right, there is Galactose. We don’t have the enzyme to break them down, but we can try. Our gut bacterial flora plays a role in this. These sugars are abundant in certain foods.
Foods with gaseous properties, such as beans and lentils, have it. We can lower the consumption of cruciferous vegetables by taking a supplement.
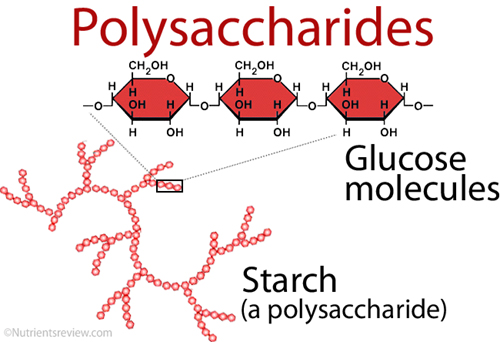
Polysaccharides:
Long polymers of monosaccharides, such as starch, glycogen, cellulose, and chitin, make up complex carbohydrates. Starch is a sugar that comes from plants and may be stored for a long time. Every human culture relies heavily on starch as a food source. It’s also a relatively simple macromolecule of numerous repeating glucose units. These are sticking together by glycosidic connections. The starch molecules in potato cells are turning into oval structures. There are also starch granules when examined under a microscope. When you add a little iodine to the potato, the starch becomes blue-black, and the granules become visible.
Read Also: Clemmensen Reduction : Explanation
Animals store starch as well, but we refer to it as glycogen. Glycogen means “sugar initiator agent.” Glycogen is a lengthy polymer of glucose, similar to plant starch. Glycogen is a type of carbohydrate that is there in our liver and muscles.
In our liver, we have around a 24-hour supply of glycogen. When we go without eating, our blood sugar drops, and the pancreas releases glucagon, a hormone that helps us feel full. Glucagon causes the liver to convert enough glycogen stores into glucose to bring our blood sugar levels back to normal. Glycogen, like plant starch, is there in the liver cells as concentrated granules.
Functions of Carbohydrates
- Carbohydrates provide immediate, short-term energy for the human body.
- Carbohydrates are a structural component of our body.
- Plants, fungus, bacteria, and arthropods use it to construct their bodies. Proteins, lipids, and nucleic acids are all made from carbohydrates.
- Plants, for example, produce glucose first and then build all of their other chemical components from it.
- In situations without glucose to meet the body’s needs, amino acids synthesize the glucose. There is also no storage molecule of amino acids. This destroys proteins, primarily from our muscle tissue. The presence of glucose spares the breakdown of proteins. Thus our body sustains itself.